Decision tree
Please select a blue box to see more information.
This decision tree will help researchers decide if they should submit an EOI and help them to be competitive.
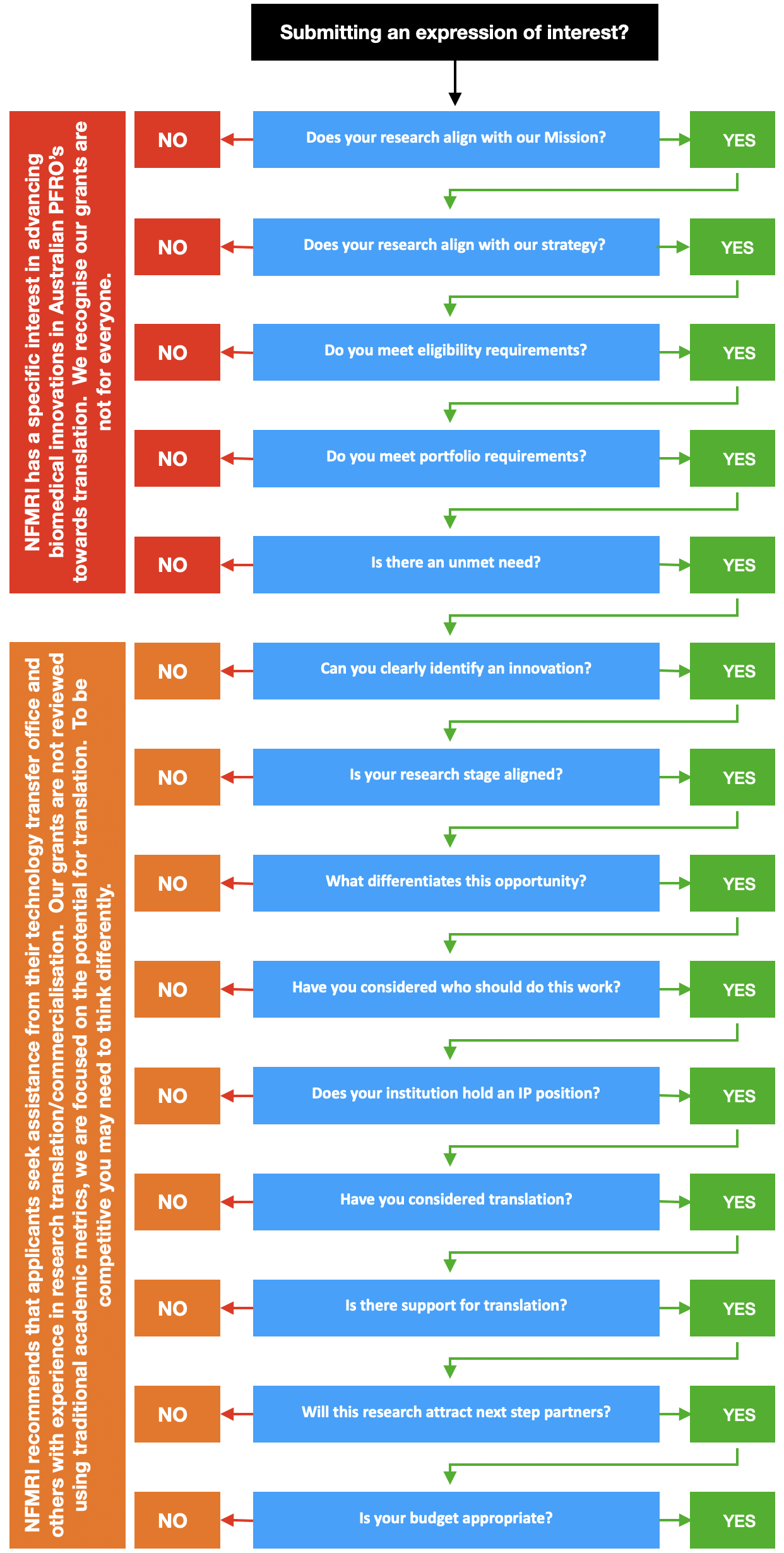
Submitting an EOI
Each year we receive many Expressions of Interest (EOI). Some of these are fantastic, and others are less competitive or not aligned with NFMRI.
Please spend the time to understand our Mission, strategy, processes and criteria. We provide plenty of information and give presentations to assist researchers.
Your time is valuable, as is that of our reviewers.
Our EOI provides the opportunity to get our attention and to be invited to submit a full application.
Our review process culls an EOI based on its weakest link. We are particularly interested in the potential for translation (research and non-research support). If you get through the cull and are invited to submit a full application, you will have the opportunity to expand on the EOI and be contacted to ensure you can address questions arising from your EOI.
Don’t forget to check out the FAQ page!
If you would like a presentation at your institution please complete the relevant form on our website.
Mission alignment
Our Mission:
“To advance innovations in medical research related to the nature, prevention, diagnosis, treatment and incidence of disease and other health problems that have a significant impact on the health of humans”.
If your research doesn’t align with our Mission, please don’t apply.
Strategic fit
Our strategy is well documented throughout our website. It is important that you read this in detail.
As a summary, we are interested in supporting preclinical biomedical research that may deliver community benefits through innovations such as new medicines, vaccines, biologicals, diagnostics, devices and tools.
The end products normally, but not always, need to eventually pass through regulatory pathways including the FDA and TGA. To achieve this, next-step partners are required.
Our strategy recognises the importance of answering key research questions to help attract quality next step partners and the need to have translation support.
If your research doesn’t strategically fit with NFMRI you should put your efforts to applying for research support that is more closely aligned.
Eligibility criteria
Please read the eligibility criteria for our grants to ensure you comply.
For clarity:
– Only researchers at Australian publicly funded research organisations (PFROs) such as universities, hospitals and medical research institutes can apply.
– The IP must be unencumbered and owned by the PFRO. First rights to negotiate are OK.
– The research NFMRI supports should be preclinical.
– The PFRO of the applicant will be awarded the grant, but research activities may be conducted by other PFROs or contract research organisations (CRO) in Australia or overseas.
– We fund research project costs only. Please don’t include travel, publication costs, CI salaries, IP costs or other expenses.
– Again, please read the full eligibility criteria before applying.
Portfolios
Please read the information on our website about our three portfolios.
We no longer accept applications for portfolio 1 in our general round. Portfolio 1 applications don’t compete well with portfolio 2 & 3 EOIs.
Portfolio 1 EOI may be available in some disease-specific rounds or partnered funding rounds, but they will compete against portfolio 2 & 3 EOIs.
A key aspect of portfolios 2 & 3 is the focus on enabling access to external capability and capacity. This enables researchers to engage with external researchers or CROs who can undertake key research activities.
Please remember that even very early research may benefit from external validation studies.
These research studies could include accessing established disease specific models, toxicology, ADME, manufacture and safety studies etc
If your research application is primarily supporting activities in your own lab, then it is portfolio 1.
Unmet needs (community benefit)
NFMRI’s mission is about delivering community benefits.
Your research should have a clear unmet need that is relevant to the grant and disease you are applying for.
What is the problem, what is currently used, why is there a need?
Does the community want it and how will they benefit?
Solutions looking for problems won’t pass this stage.
Your innovation
Before submitting an EOI you should be able to identify your potential innovation(s). Is it a drug, diagnostic or device?
We appreciate that innovations may also arise from a platform technology and that your EOI may be addressing a specific indication that may expand into other uses.
You should have an appreciation of what is currently available, how your innovation is better, what is in development and what is required to attract next-step partners.
‘Me too’ innovations are unlikely to be competitive.
Research stage
NFMRI supports preclinical research activities including research to manufacture at scale under quality systems and undertake toxicology studies of the batch so that your innovation is ready for clinical trials.
Portfolio 1 applications should NOT be basic discovery research, but early validation studies to test previous discoveries or serendipitous observations. We anticipate that any supported studies will result in data to either fail the discovery or preferably attract next-step funders such as the NHMRC.
We can and do have examples of innovations that have progressed through our three portfolios as they advance along the innovation pathway.
If you already have a commercial partner or investor, congratulations you have already crossed the ‘valley of death’ and should now be supported by them. NFMRI is not a source for undiluted capital.
Competitive advantage
Succinctly explaining your competitive advantage should differentiate your innovation and demonstrate its importance. Consider the unmet need.
You should have some evidence to support your claim. Data can also be included in the extra page of tables allowed in our EOI.
If you have safety data and other evidence of efficacy, please don’t leave it out.
The competitive advantage description in the EOI should focus on the innovation and not on the people, institution etc. even though we know this is important.
Research collaboration
A key attribute to portfolio 2 & 3 EOIs is accessing external capability and capacity.
It is important to answer the critical research questions needed by next-step partners and to have these undertaken in established labs often with quality systems in place.
If you can do it in your lab, or at your institution there are probably other funding opportunities available including internal funds from your own institution.
Sometimes the studies that are required to attract next step partners are not of interest to publishers of journals. Don’t let this stop you applying to NFMRI as we are interested in outcomes NOT outputs.
Data integrity is important to everybody in research. Quality systems and external validation can help cross the ‘valley of death’.
If successful your institute will receive the funds and need to establish agreements with your collaborators or contract research organisations.
Intellectual Property
Intellectual Property (IP) is often a key determinant for attracting next-step partners.
Be careful you don’t file too early or too late. If you have not yet filed a patent or a patent is not needed, please explain why and how you are managing the IP.
Who is managing the IP is also important.
We want to know the filing dates (provision specifications) and how the IP has progressed. What stage are your patent applications and is support continuing?
If you have a preliminary examiners report please let us know about it.
Pathways for translation
If you have little experience in translation, get help with this question.
Simple answers like ‘put on a CEO” or ‘spin out a start-up’ won’t help your EOI.
We want to know that you understand the pathway and that there is support to enable translation/commercialisation to be successful.
Please provide some detail as to the steps you have/are undertaking: if you have identified potential next-step partners, understand the market, if you know the key research questions they are needing and strategies/timing to move forward.
What scientific and other due-diligence is required and how will you address this?
Translation/commercialisation support
Part of this question could have gone at the top in red.
If your institution does NOT have a resourced knowledge transfer office, TTO or equivalent, please don’t prepare and submit an EOI.
These offices are critical for systematic translational opportunities to succeed. They can be far more than a transactional office working along side researchers to help them understand, identify and gather usable evidence to support the technology, competitive advantage, due-diligence and pre-requisite scientific studies.
We want to know what support is being given to this project and innovation. That there is a commitment from the institution and office to provide non-research capability, capacity and support to enable translation/commercialisation.
Of course evidence of support and success is helpful.
Attracting next-step partners
There are many research questions that can be asked and we all know that resources are finite.
To be effective and efficient, we encourage researchers to work with their networks and TTOs to identify potential next-step partners. To understand the key studies, methodologies, controls and quality systems that will help attract these partners is of great benefit.
They may even identify some preferred research groups or CROs where they trust results.
Knowing your consumer, working with clinicians and multidisciplinary teams can help drive success.
Budget considerations
We don’t set limits on grants as it encourages researchers to apply for what is available rather than doing the work to budget for what is needed.
We fund studies not whole projects, so that the amount requested should be for the specific studies rather than a general cost.
In some studies where milestones are relevant, we may commit tranches of funds based on success.
Grants NFMRI has committed range from less than $50,000 to $400,000.
The duration has been from less than 1 year up to 3 years.
All grants compete equally, meaning that a $400,000 grant would be compared to the benefit and need of funding 4 x $100,000 grants.
Please read our FAQs where we publish average EOI amounts and durations.
Please also note that if your budget is too large or too small for the research activities it can negatively impact the assessment.
As we also recognise that costs can change, small explainable changes in costs are permitted if invited to submit a full application. Major changes in costs and/or activities however will require a new EOI to be submitted in a subsequent round.


